The probability of a Nuclear Reaction can be defined in terms of number of particles emitted or number of nuclei undergoing transmutation for a specified number of incident particles.
It is usually expressed in terms of an effective area presented by a Nucleus towards the beam of bombarding particles, such that the number of incident particles that would strike such an area, calculated upon a purely geometrical basis, is the number observed to lead to Nuclear Reaction given in question.
This effective area is called crosssection for that reaction.
Thus the probability of occurrence of a particular Nuclear Reaction is described by effective crosssection for that process.
The crosssection may also be defined as
1) The probability that an event may occur when a single nucleus is exposed to a beam of particles of total flux one particle per unit area.
2) The probability that an event may occur when a single particle is shot perpendicularly at a target consisting of one particle per unit area.
The idea of crosssection gives imaginary area associated with each nucleus, the area is so chosen that if bombarded particle passes through it the reaction takes place, otherwise it is not.
The total nuclear crosssection is effective area possessed by a nucleus for removing incident particles from a collimated beam by all possible process.
This can be written as sum of several partial crosssections which represent contributions to various distinct, independent processes which can remove particles from incident beam.
Thus,
𝛔t = 𝛔s + 𝛔r ---------------(1)
𝛔t is "Total crosssection"
𝛔s is "Scattering crosssection"
𝛔r is "Reaction Crossection"
Scattering Crossection
Scattering crosssection can be classified as
i) Inelastic scattering
ii) Elastic Scattering
Thus, we get
𝛔s = 𝛔el + 𝛔inel --------(2)
These partial crossections can still be subdivided.
In case of elastic scattering separate partial crosssections cannot be written because of possibility of interference between them.
On other hand all inelastic scattering processses are incoherent and their crosssections are additive.
𝛔inel = 𝛔1 + 𝛔2 + 𝛔3 + ......... ------(3)
Differential crosssection
The distribution in angle of emitted particles in a nuclear
reaction can be described in terms of a crosssection which is a function of
angular coordinates in problem.
The crosssection which defines a distribution of emitted
particles with respect to solid angle is called differential crosssection. It
is defined by d
Partial crosssection for a given process is
𝛔 = ∫(d
Expression of crosssection for a Nuclear Reaction
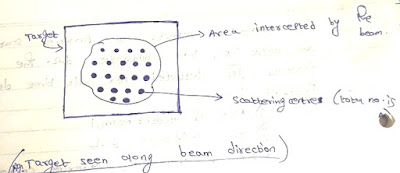
Consider a mono energetic beam of particles incident on a target shown in Fig.
Let the beam be uniform and contain ‘n’ particles per unit
volume moving with a velocity ‘V’ with respective to stationary target.
Clearly the product ‘nV’ gives number of particles crossing
a unit area perpendicular to beam per unit time. It defines flux ‘F’ of
particles in incident beam.
F = nV -------------------------------(1)
It is customary to normalize number of particles to one
particle per volume ‘V’.
n = 1/V ------------------------------(2)
The detector detects all particles scattered through an angle ‘𝛳' into solid angle dΩ.
The number of particles dN detected per unit time depends on
following factors:
i)
Flux of incident beam, F
ii) The solid angle, dΩ
iii)
Number of independent scattering centers in
target that are intercepted by the beam. Let these be N.
dN = 𝛔(𝛳)*F*dΩ*N ---------------------(3)
𝛔(𝛳) is constant of proportionality defines differential
scattering crosssection.
We can put
𝛔(𝛳) =d𝛔(𝛳) / dΩ -----------------------(4)
N = F*N*𝛔total --------------------------(5)
where,
Total Crosssection 𝛔total = ∫𝛔(𝛳) dΩ ---------------------(6)
𝛔total has dimensions of area.
Unit used to express crosssections is barn.
1 barn = 10⁻²⁸ cm².
The area 'a' intercepted by beam contains 'N' scattering centers. Total number of incident particles per unit time is given by
(Nscattered/Nincident) = (N*𝛔total)/a ------------------------(7)
𝛔total is equal to area effective in scattering for one scattering center.